Bugu da ƙari ga fasaha, haɗin glycosides ya kasance yana da sha'awar kimiyya, saboda yana da mahimmanci a cikin yanayi. Takardu na baya-bayan nan na Schmidt da Toshima da Tatsuta, da kuma nassoshi da yawa da aka ambata a ciki, sun yi tsokaci a kan nau'o'in yuwuwar roba.
A cikin kira na glycosides, an haɗa nau'ikan sukari da yawa tare da nucleophiles, irin su alcohols, carbohydrates, ko sunadarai, idan zaɓin amsawa tare da ɗayan ƙungiyoyin hydroxyl na carbohydrate da ake buƙata, duk sauran ayyuka dole ne a kiyaye su a matakin farko. A ka'ida, tsarin enzymatic ko ƙananan ƙwayoyin cuta, saboda zaɓin su, na iya maye gurbin kariyar sinadarai masu rikitarwa da matakan kariya don zaɓi daga glycosides a cikin yankuna. Duk da haka, saboda dogon tarihin alkyl glycosides, aikace-aikacen enzymes a cikin kira na glycosides ba a yi nazari sosai ba kuma a yi amfani da su.
Saboda ƙarfin tsarin tsarin enzyme da ya dace da farashin samar da kayayyaki, haɗin enzymatic na alkyl polyglycosides bai shirya don haɓakawa zuwa matakin masana'antu ba, kuma an fi son hanyoyin sunadarai.
A cikin 1870, MAcolley ya ba da rahoton haɗin "acetochlorhydrose" (1, Figure2) ta hanyar amsawar dextrose (glucose) tare da acetyl chloride, wanda ya haifar da tarihin hanyoyin haɗin gwiwar glycoside.
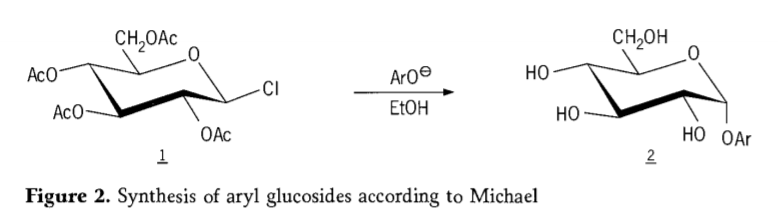
Tetra-0-acetyl-glucopyranosyl halides (acetohaloglucoses) daga baya an gano su zama masu tsaka-tsaki masu amfani don haɗakar stereoselective na alkyl glucosides. A cikin 1879, Arthur Michael ya yi nasara wajen shirya aryl glycosides na musamman daga Colley's intermediates da phenolates. (Aro-, Hoto na 2).
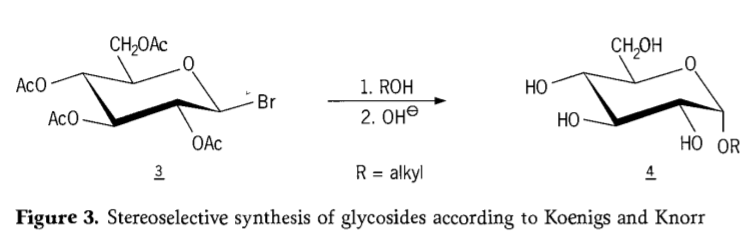
A cikin 1901, haɗin gwiwar Michael zuwa nau'ikan carbohydrates da hydroxylic aglycons, lokacin da W.Koenigs da E.Knorr suka gabatar da ingantaccen tsarin glycosidation na stereoselective (Hoto na 3). Halin ya haɗa da maye gurbin SN2 a carbon anomeric kuma yana ci gaba da stereoselectively tare da juzu'i na daidaitawa, samar da misali α-glucoside 4 daga β-anomer na tsaka-tsakin aceobromoglucose 3. Tsarin Koenigs-Knorr yana faruwa a gaban masu haɓaka azurfa ko mercury.
A cikin 1893, Emil Fischer ya ba da shawarar wata hanya ta asali daban-daban don haɗar alkyl glucosides. Wannan tsari yanzu an san shi da sunan "Fischer glycosidation" kuma ya ƙunshi halayen acid-catalyzed na glycoses tare da alcohols. Duk wani asusun tarihi ya kamata duk da haka ya haɗa da ƙoƙarin farko na A.Gautier a cikin 1874, don canza dextrose tare da ethanol mai anhydrous a gaban hydrochloric acid. Sakamakon bincike na asali na yaudara, Gautier ya yi imanin cewa ya sami "diglucose". Daga baya Fischer ya nuna cewa “diglucose” na Gautier a zahiri shine ethyl glucoside (Hoto na 4).
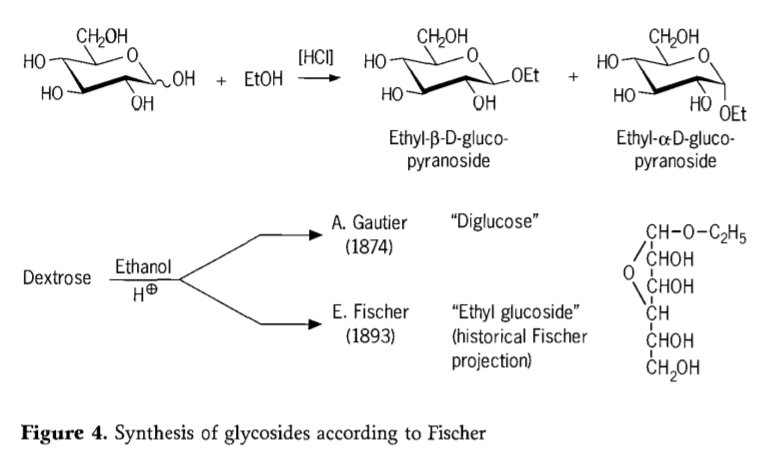
Fischer ya ayyana tsarin ethyl glucoside daidai, kamar yadda ake iya gani daga dabarar furanosidic na tarihi da aka gabatar. A gaskiya ma, Fischer glycosidation kayayyakin suna da hadaddun, mafi yawa ma'auni gaurayawan α/β-anomers da pyranoside/furanoside isomers wanda kuma ya ƙunshi bazuwar nasaba glycoside oligomers.
Saboda haka, nau'ikan kwayoyin halitta ba su da sauƙi a ware su daga gaurayawan halayen Fischer, wanda ya kasance matsala mai tsanani a baya. Bayan wasu gyare-gyare na wannan hanyar haɗin gwiwa, Fischer daga baya ya ɗauki tsarin Koenigs-Knorr don bincikensa. Yin amfani da wannan tsari, E.Fischer da B.Helferich sune farkon t bayar da rahoto game da haɗin sarkar alkyl glucoside mai tsayi wanda ke nuna kaddarorin surfactant a 1911.
A farkon 1893, Fischer ya lura daidai da mahimman kaddarorin alkyl glycosides, irin su babban kwanciyar hankali ga oxidation da hydrolysis, musamman a cikin kafofin watsa labarai mai ƙarfi na alkaline. Duk waɗannan halayen suna da mahimmanci ga alkyl polyglycosides a cikin aikace-aikacen surfactant.
Bincike da ke da alaƙa da halayen glycosidation har yanzu yana ci gaba kuma an haɓaka hanyoyi masu ban sha'awa zuwa glycosides a cikin 'yan kwanan nan. Wasu daga cikin hanyoyin haɗin glycosides an taƙaita su a cikin hoto na 5.
Gabaɗaya, ana iya raba hanyoyin glycosidation na sinadarai zuwa matakai da ke haifar da hadaddun ma'aunin oligomer a cikin musayar glycosyl acid-catalysed.
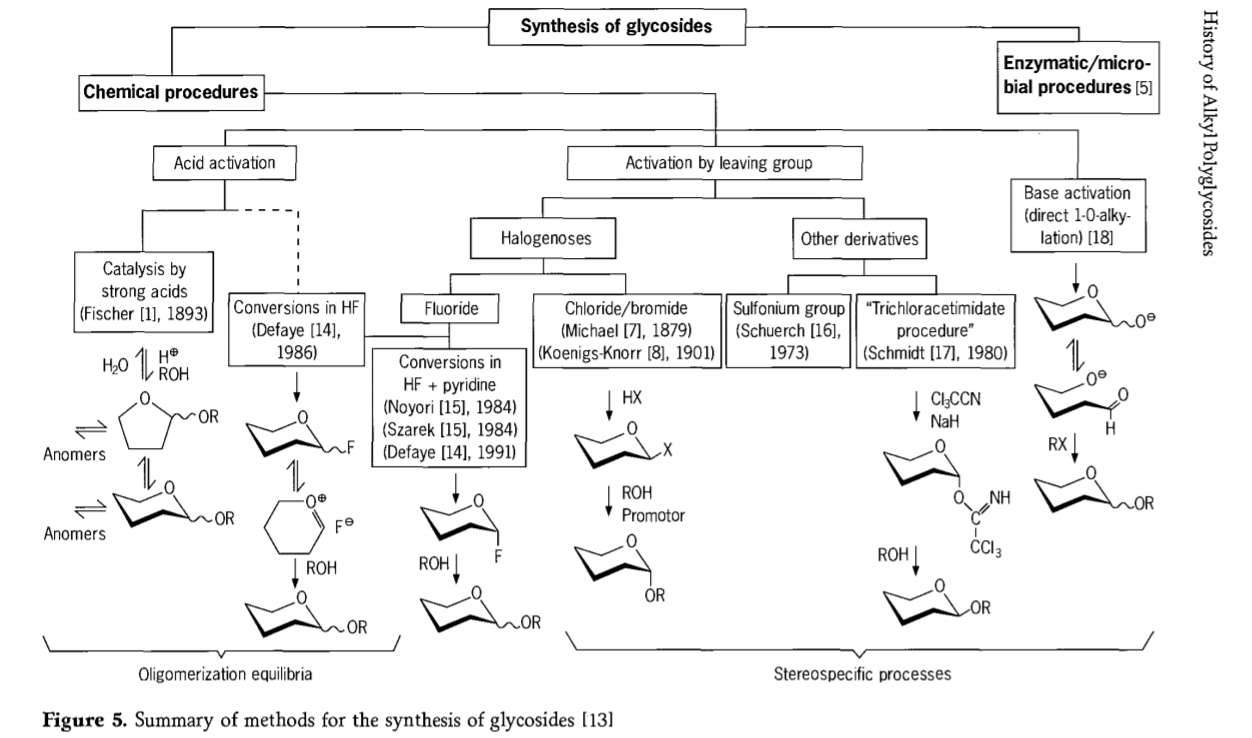
Abubuwan da suka dace akan abubuwan da aka kunna carbohydrate mai dacewa (halayen Fischer glycosidic da halayen hydrogen fluoride (HF) tare da kwayoyin carbohydrate mara kariya) da motsin motsin rai, wanda ba zai iya jurewa ba, kuma galibi halayen maye gurbin stereotaxic. Nau'in hanya na biyu na iya haifar da samuwar nau'ikan mutum ɗaya maimakon a cikin hadaddun halayen halayen, musamman idan aka haɗa su tare da dabarun ƙungiyar kiyayewa. Carbohydrates na iya barin ƙungiyoyi akan carbon ectopic, kamar su halogen atom, sulfonyls, ko ƙungiyoyin trichloroacetimidate, ko kuma a kunna su ta tushe kafin juyawa zuwa esters triflate.
A cikin yanayi na musamman na glycosidations a cikin hydrogen fluoride ko a cikin gaurayawan hydrogen fluoride da pyridine (pyridinium poly [hydrogen fluoride]), glycosyl fluorides suna samuwa a cikin wurin kuma suna jujjuya su cikin glycosides, misali tare da barasa. An nuna sinadarin hydrogen fluoride a matsayin mai ƙarfi mai kunnawa, matsakaicin amsawa mara lalacewa; Ana lura da ma'auni auto condensation (oligomerization) kama da tsarin Fischer, kodayake tsarin amsawa yana iya bambanta.
Kemikal tsarkakakkun alkyl glycosides sun dace kawai don aikace-aikace na musamman. Misali, an yi amfani da alkyl glycosides cikin nasara a cikin bincike na biochemical don crystallization na sunadaran membrane, irin su crystallization na porin da bacteriorhodopsin a gaban octyl β-D-glucopyranoside (ƙarin gwaje-gwajen dangane da wannan aikin ya kai ga lambar yabo ta Nobel a cikin ilmin sunadarai ga Deisenhofer, Huber da Michel a cikin 1198).
A yayin ci gaban alkyl polyglycosides, an yi amfani da hanyoyin stereoselective akan sikelin dakin gwaje-gwaje don haɗa nau'ikan samfuran samfuri da kuma nazarin kaddarorinsu na physicochemical, saboda rikicewarsu, rashin zaman lafiya na tsaka-tsaki da adadin da mahimmancin yanayin aiwatar da ɓarna, haɗuwar nau'in Koenigs-Knorr da sauran manyan matsalolin tattalin arziki da fasaha zai haifar. Hanyoyin nau'in Fischer ba su da rikitarwa kuma suna da sauƙin aiwatarwa a kan sikelin kasuwanci kuma bisa ga haka, sune hanyar da aka fi so don samar da alkyl polyglycosides a kan babban sikelin.
Lokacin aikawa: Satumba 12-2020





