Halayen Physicochemical na Alkyl Polyglycosides-Habiyar Hali
Tsarin binary
Tsarin tsari na C12-14 alkyl polyglycoside (C12-14 APG)/ tsarin ruwa ya bambanta da na APG gajeriyar sarkar. (Hoto na 3). A ƙananan yanayin zafi, an kafa wani yanki mai ƙarfi / ruwa a ƙarƙashin madaidaicin Krafft, yana kan kewayon taro mai faɗi. Tare da karuwar yawan zafin jiki, tsarin yana canzawa zuwa wani lokaci na ruwa na isotropic. Saboda crystallization an jinkirta kinetically zuwa wani babba iyaka, wannan lokaci iyaka canza matsayi tare da ajiya lokaci. A low yawa, isotropic ruwa lokaci canje-canje sama da 35 ℃ a cikin wani kashi biyu-lokaci yanki na biyu ruwa bulan, kamar yadda aka saba gani tare da nonionic surfactants. A ƙididdigewa sama da 60% ta nauyi, jerin lokaci na crystalline na ruwa yana samuwa a duk yanayin zafi. Yana da kyau a ambaci cewa a cikin yanki guda ɗaya na isotropic, ana iya lura da birefringence na fili a fili lokacin da maida hankali kawai ya kasance ƙasa da narkar da lokaci, sa'an nan kuma ya ɓace da sauri bayan an kammala aikin shear. Koyaya, ba a sami wani yanki na polyphase da ya rabu da matakin L1 ba. A cikin lokacin L1, wani yanki mai rauni mai raɗaɗi yana kusa da mafi ƙarancin ƙimar tazarar ruwa/ruwa.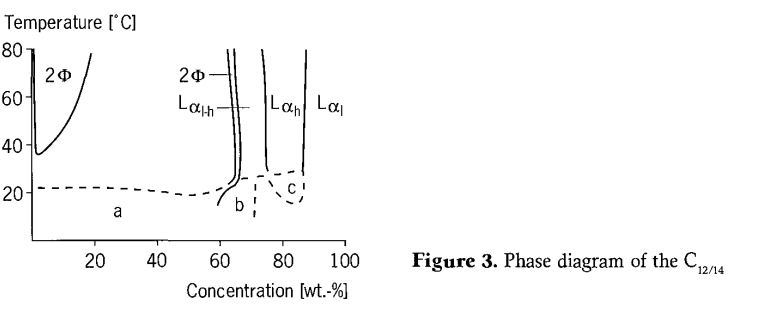
Platz et al ne suka gudanar da binciken phenomenological game da tsarin matakan kristal na ruwa. Amfani da irin waɗannan hanyoyin kamar polarization microscope. Bayan waɗannan binciken, ana la'akari da yankuna uku daban-daban a cikin mahimmin hanyoyin C12-14 APG: Lαl ,LAlhda LA. Akwai nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan nau'ikan guda uku bisa ga ƙayyadaddun ƙira.
Bayan an adana shi na dogon lokaci, lokaci mai mahimmanci na ruwa na lamellar crystalline yana haɓaka yankunan pseudoisotropic duhu a ƙarƙashin haske mai haske. Waɗannan yankuna an raba su a fili daga wuraren da ba su da ƙarfi sosai. Lokaci na Lαh, wanda ke faruwa a cikin matsakaicin matsakaicin kewayon yanki na ruwa crystalline, a yanayin zafi mai girma, yana nuna irin wannan laushi. Ba a taɓa ganin nau'in nau'in nau'in Schlieren ba, kodayake ana samun ratsi mai ƙarfi mai ƙarfi. Idan samfurin da ke ɗauke da wani lokaci na Lαh ya sanyaya don sanin ma'anar Krafft, rubutun yana canzawa a ƙasa da yanayin zafi. Yankunan pseudoisotropic da raƙuman mai da aka bayyana a sarari sun ɓace. Da farko, babu C12-14 APG crystallizes, a maimakon haka, wani sabon yanayin lyotropic wanda ke nuna raunin birefringence kawai an kafa. A in mun gwada da babban taro, wannan lokaci yana faɗaɗa har zuwa yanayin zafi. A cikin yanayin alkyl glycosides, wani yanayi daban-daban ya fito. Duk masu amfani da wutar lantarki, ban da sodium hydroxide, sun haifar da raguwa mai mahimmanci a cikin wuraren girgije.The maida hankali kewayon electrolytes ne game da wani tsari na girma kasa da na alkyl polyethylene glycol ethers.Abin mamaki, akwai kawai sosai kadan bambance-bambancen da ke tsakanin mutum Cloudalkaliness. Don bayyana bambance-bambancen halaye tsakanin alkyl polyglycol ethers da alkyl polyglycol ethers, ana ɗauka cewa ƙungiyar OH da aka tara a cikin rukunin glucose ta sami nau'ikan hydration daban-daban tare da ƙungiyar ethylene oxide. Mafi girman tasirin electrolytes akan Alkyl polyglycol ethers yana nuna cewa akwai caji akan saman alkyl polyglycoside micelles, yayin da alkyl polyethylene glycol ethers ba su da wani caji.
Don haka, alkyl polyglycosides suna nuna kamar gaurayawan alkyl polyglycol ethers da anionic surfactants.Nazarin hulɗar tsakanin alkyl glycosides da anionic ko cationic surfactants da ƙaddarar yuwuwar a cikin emulsion ya nuna cewa alkyl glycosides micelles suna da cajin mara kyau a cikin kewayon pH 3 polyky. glycol ether micelles yana da rauni tabbatacce ko kusa da sifili. Dalilin da yasa ake cajin miceles na alkyl glycoside ba a cika yin cikakken bayani ba.
Lokacin aikawa: Oktoba-22-2020





